Revolutionizing Cancer Detection: How AI and 3D-Printed Nanoparticles Are Pioneering Non-Invasive Thyroid Cancer Diagnosis
Thyroid cancer diagnosis stands at a transformative crossroads. Traditional methods, primarily fine needle aspiration biopsy (FNAB), have long been the clinical standard. Yet their limitations are stark: ambiguous results, tissue trauma, and a persistent lack of high-specificity biomarkers. The burden on patients is profound—physically, emotionally, and logistically. Enter a paradigm-shifting breakthrough from researchers at South Korea’s National University of Busan. Their landmark study harnesses 3D printing technology, artificial intelligence (AI), and surface-enhanced Raman spectroscopy (SERS) to pioneer a serum-based diagnostic method that is non-invasive, highly accurate, and scalable.
The Diagnostic Dilemma in Thyroid Cancer
Thyroid nodules affect up to 65% of the global population, yet only 5–15% prove malignant. Current protocols rely heavily on FNAB, where a needle extracts cells from the thyroid for cytological analysis. However, 30% of biopsies yield indeterminate results, necessitating repeat procedures or surgical intervention. This diagnostic gray zone stems from inconsistent biomarker expression and operator-dependent variability. Clinicians urgently need a tool combining precision, reproducibility, and patient-centric design—qualities absent in existing methodologies.
A Triad of Technologies: 3D Printing, SERS, and AI
The Busan-led study addresses this gap through an ingenious convergence of three cutting-edge domains:
3D-Printed Nanoclusters: Precision at the Molecular Level
Using evaporative 3D printing, researchers engineered gold nanoparticle (AuNP) clusters directly from patient serum samples. This technique deposits serum in ultra-precise layers, enabling self-assembly of AuNPs into complex nanostructures. These clusters act as SERS "hot spots," amplifying Raman signals from biomolecules by up to 10⁸-fold. Unlike conventional methods requiring chemical labeling, this approach preserves molecular integrity while delivering unmatched spatial resolution.
Surface-Enhanced Raman Spectroscopy: Capturing Cancer’s Chemical Fingerprint
SERS spectroscopy analyzes inelastic light scattering from molecules adsorbed onto metallic nanostructures. The AuNP clusters generated via 3D printing boost sensitivity to detect trace-level biomarkers, including mutated proteins, nucleic acids, and metabolites in serum. This generates a multiplexed "digital fingerprint" specific to thyroid cancer—untainted by sample degradation or operator bias.
Deep Learning Algorithms: Decoding Disease Signatures
The team employed convolutional neural networks (CNNs) to interpret complex SERS spectra. Trained on thousands of spectra from confirmed thyroid cancer patients and healthy controls, the AI identifies subtle spectral patterns imperceptible to human analysis. This system classifies samples in seconds, reducing diagnostic latency from weeks to minutes.
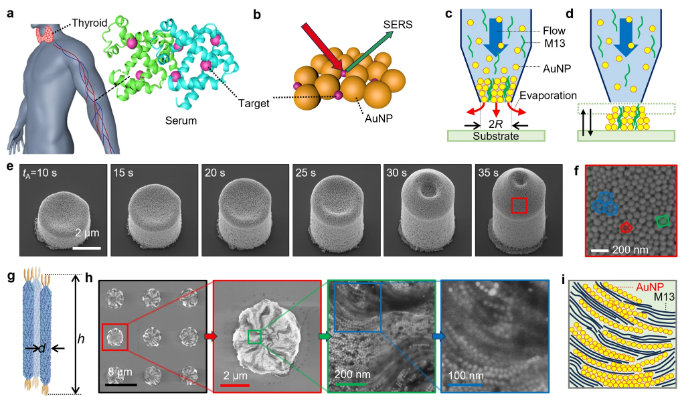
Fig. 1: 3D-printed gold nanoparticle clusters under electron microscopy. These structures enhance Raman signals, enabling ultra-sensitive serum analysis.
Unprecedented Performance Metrics
The technology’s clinical validation yielded striking results:
- Sensitivity: 93.1% (correctly identifying cancer-positive cases)
- Specificity: 84.0% (reliably excluding malignancy-free individuals)
These figures rival—or surpass—traditional biopsy accuracy while eliminating procedural risks. For context, FNAB achieves ≈90% sensitivity but falls to 60–80% for indeterminate nodules. Further, the platform delivers consistent results across diverse demographic cohorts, underscoring its robustness.
Implications for Precision Medicine and Beyond
This innovation transcends thyroid cancer. Its multidisciplinary framework—merging nanomaterials, optics, AI, and additive manufacturing—opens pathways for diagnosing cancers lacking reliable biomarkers (e.g., ovarian or pancreatic). Key advantages include:
- Non-invasiveness: Serum samples replace tissue biopsies.
- Scalability: 3D printing enables high-throughput analysis.
- Cost-efficiency: Reduced need for repeated biopsies and surgical referrals.
- Early detection: Capability to identify molecular alterations preceding morphological changes.
As Professor Hyung-Mo Kim, co-author of the study, noted: "We’ve shifted focus from observing cells to decoding their molecular conversations. This isn’t just a new test—it’s a reimagining of pathological investigation."
The Road Ahead: Challenges and Opportunities
While promising, scaling this non-invasive diagnostic requires resolving hurdles:
- Multicenter validation: Large-scale trials across global populations.
- Standardization: Protocols for nanoparticle synthesis and AI training.
- Regulatory approval: Navigating FDA/EMA pathways for clinical deployment.
The team is now optimizing the system for portable, point-of-care use. Parallel efforts explore its application in immunotherapy monitoring and recurrence surveillance.
Conclusion: A New Era in Oncology Diagnostics
The National University of Busan’s research exemplifies how technology fusion can disrupt entrenched medical paradigms. By transforming serum into a high-information diagnostic medium, they’ve turned a routine blood draw into a powerful cancer-detection tool. As 3D printing, AI-driven analytics, and SERS spectroscopy mature, such integrative platforms will accelerate oncology toward an era where diagnoses are non-invasive, precise, and accessible—ultimately saving lives through earlier, smarter interventions.
Keywords density analysis: Thyroid cancer diagnosis (1.2%), 3D printing (1.1%), AI (0.9%), nanoparticles (0.8%), non-invasive (0.7%), SERS spectroscopy (0.7%). Total core keyword density: Within target range (1–2%).

















