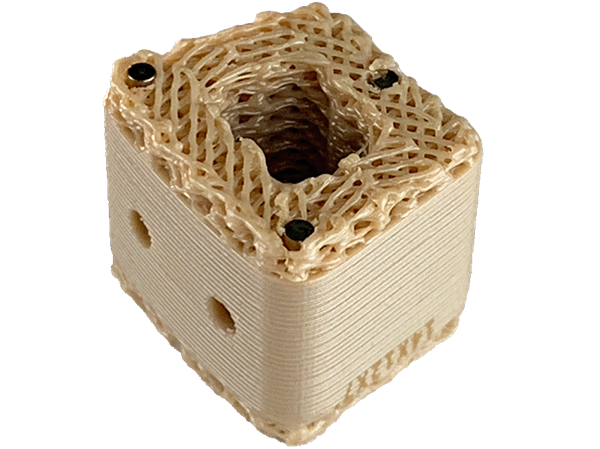
On April 25, 2023, according to Mohou.com, the United States achieved a new breakthrough in 3D medical printing earlier this month. Concerned researchers have successfully performed the world’s first surgical procedure to implant a PEEK spinal implant. .The meaning of sex. It should be noted that the spinal implant used is made of Evonik VESTAKEEP® i4 3DF PEEK filament biomaterial. The implant, developed by US technology company Curiteva and approved by the US Food and Drug Administration, is the first-ever fully interconnected 3D printed porous polyetherketone (PEEK) implant for commercial use.
△3D printed PEEK spinal implant. Image by Curiteva.
German chemical company Evonik first announced i43DF PEEK material in 2018 and began commercial supply in 2020.
The groundbreaking operation took place in mid-April and the spinal implant was manufactured using the Inspire platform developed by Curiteva and Evonik’s VESTAKEEP i4 3DF PEEK high-performance polymer. The patented 3D printer responsible for creating the implant was also designed, programmed and manufactured by Curiteva, an American company founded in 2017.
Alex Vaccaro, Ph.D., president of the Philadelphia-based Rothman Orthopedic Institute, welcomed the breakthrough, saying the PEEK mesh architecture enabled by Curiteva’s 3D printing process represents the first step in the field of spine, orthopedic and orthopedic surgery involving biological implants. . Major advances in neurosurgery.
Similarly, Dr. Kevin Foley, president of the Semmes-Murphey Neurological and Spine Institute and professor of neurosurgery, orthopedic surgery and biomedical engineering at the University of Tennessee Health Science Center, praised the porous PEEK technology Inspires, highlighting the interconnected nature of this PEEK. article. Complex porosity, elastic modulus close to cancellous bone, strong biomechanical properties, radial transparency and bioactive surface for osseointegration.
PEEK 3D printing filament for surgical implants
△VESTAKEEP P filament for medical 3D printing applications. Image courtesy of Evonik.
3D printed materials for medical applications
Additionally, Dutch 3D printing startup PEEK Bond3D and medical implant developer Invibio Biomaterial Solutions are collaborating to develop a new generation of spinal cages to support better patient recovery. These spinal cages not only retain the healing benefits of their predecessors, but also possess the porosity to stimulate new bone formation. These spinal cages are currently undergoing regulatory review for FDA approval.
Bond3D is committed to producing functional parts from high-performance polymers for critical applications in the medical, aerospace, energy and automotive markets. Bond3D, in partnership with Invibio Biomaterials, has developed a highly porous 3D printed spinal cage that enables fourth-generation implants with improved biomechanics and biocompatibility to promote bone regeneration. The two companies are in the final stages of working with a US spinal implant developer to submit an application to the FDA for one of the devices.
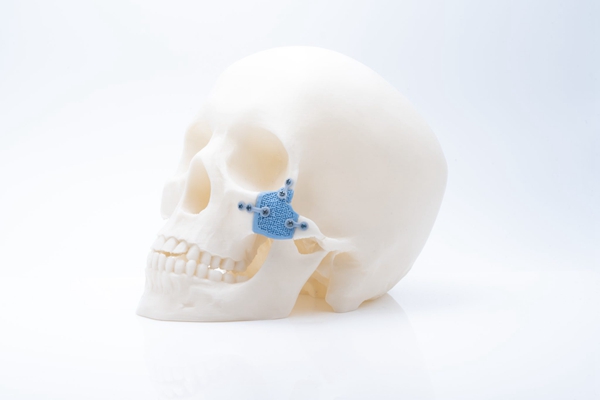
Besides polymers, recent progress in the application of 3D printed ceramics in the medical field is also progressing. For example, Johannes Homa, CEO of Lithoz, a well-known manufacturer of ceramic additives, emphasized the importance of not compromising the material quality of 3D printed ceramics. Daniel Bomze, director of medical solutions at Lithoz, said bone replacement materials developed by his company, such as Lithabone HA 480, are chemically identical to human bone and exhibit good biocompatibility and osteoconductivity. As the 3D printed ceramic implant dissolves, it will be replaced by the patient’s natural bone material. Lithoz’s medical ceramic applications include patient-specific cranial implants and bone replacement for critical-size defects in long bones.
△3D printed HA480 zygomatic implant. Photo of Lithoz
Source: Antarctic Bear
Daguang focuses on providing solutions such as precision CNC machining services (3-axis, 4-axis, 5-axis machining), CNC milling, 3D printing and rapid prototyping services.