Link3D is a company with patented manufacturing technology that enables the use of high-performance polymer functional parts, and recently3DHigh resistance printing AT A GLANCE Significant progress has been made in maturing component technology with performance comparable to injection molded or machined components.AT A GLANCEThe parts are comparable. The company has now joined forces with Invibio Biomaterials Solutionscollaborate to develop the next generation ofAT A GLANCEspinal fusion device.
© Bond3D
High strength, porousAT A GLANCEimplant
The medical device industry willAT A GLANCEFor long-term orthopedic, spinal, trauma and cardiovascular implants,AT A GLANCE The ability to bring clinical and economic benefits to medical devices has resulted in more than1500million patients accepted AT A GLANCE implants, whileLink3D‘s technology facilitates the development of other solutions for medical devices by enabling the creation of high-strength porous implants that enable bone growth, in addition to rapid growth3DPrint patient-specific implants.
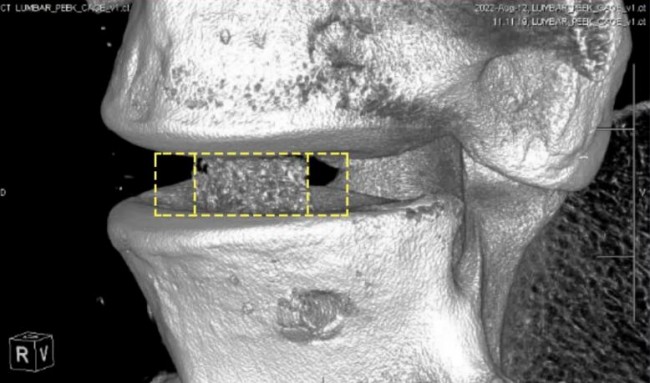
3D linkPrinted spinal cageCTpicture© Bond3D
One of the biggest opportunities for porous implants lies in the area of spinal interbody fusion devices. They are used to treat spinal problems in more than a million patients each year. When designing interbody fusion devices, the industry wants to produce devices that allow. for an artifact-free passage. CT/MRI Image evaluation fusion device. In addition to including highly porous areas with the correct functionality to promote bone growth, thereby reducing the risk of cage subsidence by producing implants with a modulus similar to that of natural bone, it is necessary to develop titanium spinal fusions that are more porous than the current generation. more advanced solutions.

Valley_Implantsimplant3Dprinting technology© 3DScience Valley White Paper
NOW,Link3D The necessary capabilities have been established to design highly porous materialsAT A GLANCEspinal cage to meetFDABiomechanical and biocompatibility requirements required for approval.
AT A GLANCE Biocompatibility of materials
Scientific research shows that bone remodeling is very sensitive to dynamic loading. Due to the stress shielding effect, it is necessary to research and develop materials with a lower elastic modulus than metal materials, as well as high short-term and long-term mechanical strength for orthopedic implants.
AT A GLANCEThe material has become an excellent material for orthopedic implants due to its stable chemical properties, high structural strength and respect for bone mechanical properties. The material has been widely used in mechanical support applications in the orthopedic field, such as spinal, thoracic, lumbar and cervical, orthopedic and trauma implants.
However, it has disadvantages such as processing difficulty and high biological inertia, which limits the design and application of this material. How to improve the biosafety, biocompatibility, osteogenic effect and other biological activities of polyetheretherketone implant materials is an important breakthrough to further expand the application of this material.
according to3DUnderstanding the market of Science Valley, in this regard in China, Guo Zheng and Li Xiaokang of the Fourth Military Medical University and Zheng Yufeng’s research team of Peking University prepared the product through the technology of deposition by fusion casting.3DPorous printingAT A GLANCEimplants, on which polydopamine (PDA) coating and usePDAChelates biologically active magnesium ions. The research team proved3DPorous printingAT A GLANCEstructure and build on its surfacePDA-Mg2+Bioactive coatings provide a simple way to improve the biocompatibility of bioinert materials.
hard tissue implants
according to3DValley of Sciences3DPrint–In the article “Overview of Advances in Additive Manufacturing of New Materials, Medical Devices, and Related Regulatory Scientific Research”, in recent years, polyetheretherketone has3DApplications in the field of printing hard tissue substitutes have received wide attention. This polymer material has excellent biocompatibility and chemical stability, and its density and mechanical properties are close to those of human bones. It is an ideal bone substitute material.3DThe combination of printing technologies is expected to be widely used in the field of orthopedic implants.
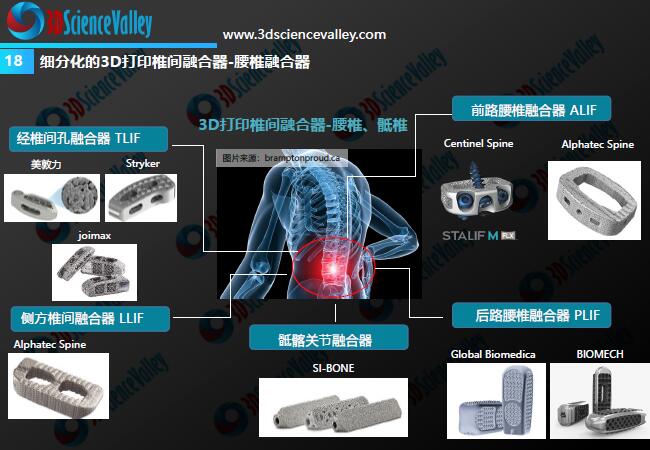
© 3DScience Valley White Paper
Hard tissue substitutes are one of the fastest growing areas in the additive manufacturing medical device industry in recent years, and international orthopedic medical device giants have created this huge market. our country is in2018The first custom-made, additively manufactured titanium mandibular replacement was approved in 2016, with subsequent approvals3DPrint titanium alloy acetabular cups, titanium alloy intervertebral fusion cages and other products. Xi’an Jiaotong University is located3DInnovative technical and application research has been carried out on the printing of polyetheretherketone bone substitutes.2017Since the start of the year, this has gradually come to fruition3DClinical applications of printing medical devices such as thoracic ribs, mandible and skull.
domesticAT A GLANCEof3DIn terms of printing equipment and materials,INTAMSYSIntelligent Yuanzhu in high performance materials (material extrusion)3DThe printing company continues to expand its product line to includeFUNMATIndustrial series3DPrinting equipment and its adapted high performance3D PEEKPrinting supplies.
Source: 3D Science Valley
Daguang focuses on providing solutions such as precision CNC machining services (3-axis, 4-axis, 5-axis machining), CNC milling, 3D printing and rapid prototyping services.