On April 7, 2023, Mohou.com learned that medical 3D printing company restor3d announced that the United States Food and Drug Administration (FDA) granted the company 510(k) certification of the Axiom PSR system, which is a system based on implantable medical devices. using metal 3D printing technology are used to treat orthopedic diseases.

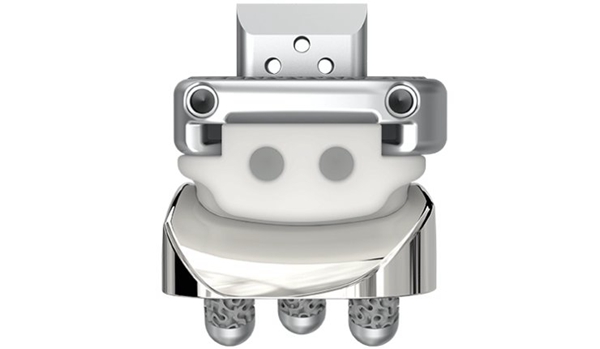
△3D printed implants for ankle surgery made by restor3d
The latest addition to 3D printed orthopedic implants
The Axiom PSR System is the latest addition to restor3d’s lower extremity product line and is additively manufactured from a titanium alloy with integrated interconnect porosity from TIDAL technology. It is the first all-metal, patient-specific instrumentation system approved for use with an ankle replacement system.
The system is composed of several product segments, including the Kinos Axiom total ankle system, the TIDAL osteotomy wedge system and the MTP hemiarthroplasty system, as well as the r3id custom surgical platform. The integration of these components allows the Axiom PSR System to provide more precise total ankle surgery and can be designed and performed individually based on each patient’s unique situation.
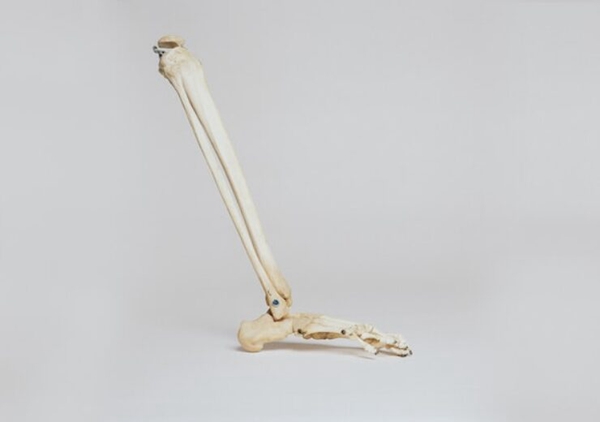
3D printed ankle implant from △restor3d
Axiom PSR Full Metal Surgical Ankle System
According to the company, the Kinos Axiom system was designed in collaboration with operative surgeons to provide precision, accuracy and efficiency during total ankle surgery.
The all-metal resection guide is 3D printed at Restor3D’s Durham, North Carolina facility, ensuring ideal anatomical fit and precise placement of the pre-planned incisions required for the Kinos Axiom Total Ankle System implant. The simplified design of the system aims to reduce operating time during total ankle surgery.
The Axiom PSR guide uses a proprietary surface topography to increase the stability of the bone contact interface. The all-metal resection guide also incorporates radiographic markers for easy fluoroscopic visualization and has a small footprint to reduce soft tissue trauma.
△restor3d uses the Kinos Axiom total ankle system to treat ankle arthritis
“The Axiom PSR Metal Resection Guide sits better on the bone and provides greater control over the path of the saw than patient-specific polymer instruments of the past,” said John Lewis, MD, of Louisville Orthopedic Clinic. PSR, by cutting the guide. allows him to make repeatable cuts for total ankle surgery with greater confidence while also reducing his time in the operating room. Additionally, the guide incorporates radiographic markers and reduces soft tissue disruption, along with other benefits.
Brian Garvey, Senior Vice President of Product Development at restor3d, added: “We are extremely excited to work with the team to achieve 510(k) clearance for the first specific all-metal arthroplasty instrumentation system to the patient. capabilities, advanced engineering Our collaboration with a clinically focused research team allows us to deliver the next generation of patient-specific devices. Patient-specific development programs lay the foundation.
Axiom PSR will launch a trial version in April this year and will be officially launched in June 2023.
Source: Antarctic Bear
Daguang focuses on providing solutions such as precision CNC machining services (3-axis, 4-axis, 5-axis machining), CNC milling, 3D printing and rapid prototyping services.