On September 22, 2022, the team of Director Chen Fangtian of the Spine Surgery Department of Xiangya Changde Hospital successfully performed a case of cervical disc nucleectomy + partial C5 vertebral body resection + anterior cervical spinal canal decompression + exploration of spinal nerve roots + 3D printed porous. placement of the vertebral body fusion cage. The porous vertebral body cage was developed and manufactured by Hunan Huaxiang Medical Technology Co., Ltd. and the Spine Surgery Team of the First Affiliated Hospital of South China University using metal 3D printing solutions. It has obtained a Class III medical device license. using selective laser melting technology (SLM metal 3D printing technology) of orthopedic implant prosthetic products.
△3D Printed Metal Porous Vertebral Body Fusion Cage
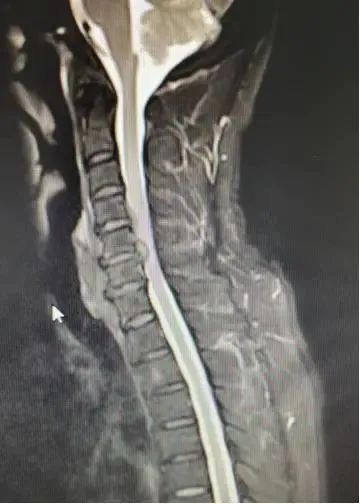
Patient Chen XX, a 51-year-old man, reported having neck pain with no obvious trigger eight months ago. The pain got worse after exercise. He underwent acupuncture, cupping and other treatments, and the symptoms were relieved during this time. I still felt pain intermittently. Special treatment was administered. During this period, the above symptoms worsened and numbness and discomfort appeared in the limbs, mainly in the index and middle fingers of the right hand, as well as five fingers of the left hand, under the knee joint. of the right lower limb and on the sole of the left lower limb, accompanied by weakness of the left lower limb. In order to seek further treatment, the patient reported to Xiangya Changde Hospital for treatment. The outpatient department was admitted to the spine surgery department with the diagnosis of “cervical spondylotic myelopathy”.
After the patient was admitted to the hospital, relevant examinations were carried out. Director Chen Fangtian examined the patient’s condition in detail and said: The patient presented with cervical disc herniation (C4/5, C5/6), cervical spinal canal stenosis (C5/6). ), and symptoms of spinal cord and nerve compression. The diagnosis was clear, non-surgical treatment is ineffective, the patient has clear indications for surgery and has no obvious contraindications to surgery, therefore surgical treatment can be considered.
△Patient preoperative imaging data
After discussions within the department and communication with patients and their families, Director Chen Fangtian’s team used the 3D printed porous spinal fusion device designed and produced by Hunan Huaxiang Medical Technology Co., Ltd. to resolve the patient’s problem.
Compared to traditional titanium mesh, metal 3D printed porous spinal fusion cages have three major advantages:
Firstly, based on the patient’s preoperative imaging data, personalized adaptation of the prosthesis is carried out to adapt the prosthesis to the structure of the vertebral endplate, which can maximally restore and reconstruct the physiological curvature of the spine. vertebral.
Secondly, the end surface of the prosthesis is designed with a curved anatomical surface to increase the contact surface of the vertebral endplate and avoid stress concentration, which can ensure good stability and reduce postoperative complications such as subsidence. and loosening of the prosthesis.
Third, the 3D bone-like trabecular structure design is used. The porous structure has good permeability, and bone tissue can grow effectively in the porous structure, thereby improving the bone fusion rate.
The operation was carried out successfully. The muscle strength of the patient’s limbs improved significantly, and the pain and numbness were relieved after the operation. Postoperative imaging showed adequate decompression and good cage position. The patient was very satisfied with the surgical results.
Postoperative Patient Imaging Data
As a key research and development result of the 13th Five-Year Plan, the porous spinal fusion cage is made of titanium alloy powder (Ti6AI4V) as raw material. It is the first domestic model to be molded using 3D printing selective laser melting (.SLM) technology, and has obtained Class III medical device certification.

The vertebral body matches the anatomical angle of the upper and lower vertebral endplates, has strong adaptability, and fully meets clinical needs. The design of large bone graft window promotes the fusion effect of bone graft. Structurally, it adopts a simulated bone trabecular structure design, and its elastic modulus is similar to that of human bone tissue, which can effectively reduce the risk of internal implant collapse. Its porosity is 80%±, the pore structure is 300μm±, and the wire diameter is 200μm±, which can provide a good spatial environment for the migration and proliferation of bone cells, thereby achieving growth bone, completing osseointegration and obtaining a long lifespan. -term stable state.
source:
Daguang focuses on providing solutions such as precision CNC machining services (3-axis, 4-axis, 5-axis machining), CNC milling, 3D printing and rapid prototyping services.