On television, you may have seen a blacksmith dipping a red-hot sword in water or oil. This is called “extinction” (don’t feel bad if you think it’s called “extinction”, it’s a common mistake). Quenching is an ancient method of rearranging the atomic structure of a material.
Quenching is the process of rapidly cooling a material, usually a metal, to achieve desirable mechanical properties such as increased strength and hardness.
Most people think of quenching as simply immersing hot steel in a bucket of water, but materials scientists can quench it in water, oil, liquid nitrogen, and even air . Quenching is an important processing step for many materials. Quenching can refine grains or manipulate phase transitions. A very important phase transition is that from martensite to austenite in steel, which I will discuss in depth later.
To determine the effect of quenching on a metal, materials scientists can use tests or time-temperature-transition (TTT) curves.
We’ll come back to these technical terms later, but first let’s look at all the cool things you can do with tempering.

In this article, we will focus on the following points to learn how to “quench” with each one:
1. What is the purpose of quenching?
2.What is the history of tempering?
3. What is a quenching medium?
4. What happens when steel is quenched?
5. What are the characteristics of the martensitic transformation?
6. What are the main characteristics of martensite?
7. What is retained austenite?
8. How does carbon content affect the hardness of quenched steel?
9. What are the stages of quenching?
10. How fast do we need to quench to get martensite (what is a TTT curve)?
11. Final Thoughts
1. What is the purpose of quenching?
When most people think of quenching, they probably imagine a blacksmith placing a piece of red-hot steel into a bucket of water. The spectrum of quenching is much broader than that, but let’s start with this example and explore what quenching can do in steel.
The first thing quenching can do is refine the grain.
If you didn’t know, metals are crystals. Unlike the crystals you usually think of, like gemstones, metals are “polycrystalline.” This means that the metal is made up of many small crystals. We call each of these little crystals a “particle.”
It turns out that repeating patterns are a very stable way for atoms to organize themselves. The three most common crystal structures are body-centered cubic (BCC), face-centered cubic (FCC), and hexagonal close-packed (HCP).
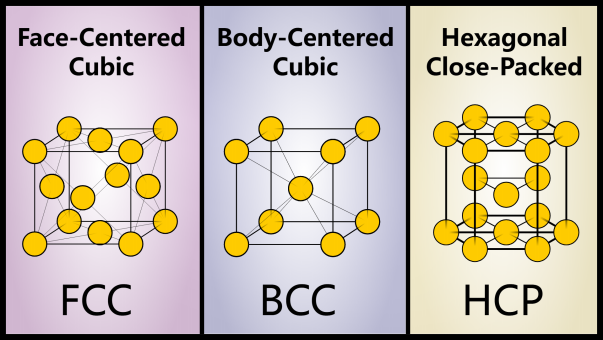
The way atoms are arranged can have a huge impact on the properties of materials – just look at diamond and graphite! Much of materials science affects the properties of materials by changing the crystal structure.
When a layman talks about crystals, they are usually referring to precious stones: diamonds, rubies, sapphires or salt. This is a particular type of crystal: a single crystal. A single crystal has an unbroken pattern of atoms throughout the material.
Most often, materials such as metals are polycrystalline. These materials still have a repeating structure, but this structure is broken down into different grains.
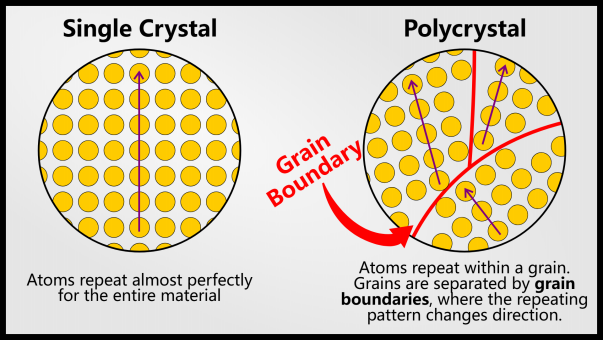
When a phase solidifies, it starts with just a few atoms. These atoms come together and grow. All atoms joining this group will have the same orientation and will form a particle. At some point, one grain will collide with another.
If the phase solidifies more slowly, there will be fewer initial grains and each grain will be larger. If the phase solidifies more quickly, there will be many initial grains, each smaller.
Generally speaking, smaller grains mean a stronger, less ductile metal. I hope I’ve illustrated this with my homemade animation, but if you want to look it up yourself, it’s called the Hall-Page effect.
Now, in my explanation, I may be implying something incorrect. This pattern of grain nucleation and growth looks very interesting when you create a solid phase from a liquid, but it is not typical quenching.
For example: In steel, quenching takes us from one solid phase to another. This still results in grain refinement, but it also has larger implications.
Steel begins in a phase called “ferrite”. When you heat steel, it transitions to a new phase called “austenite.” Austenite can dissolve more carbon than ferrite, which is a good thing. If you cool the austenite you will get ferrite again – provided you cool the steel slowly enough to give the carbon time to leave the metal.
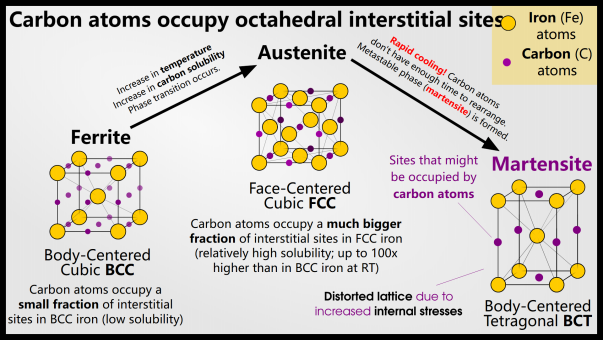
On the other hand, if you cool the steel quickly, you obtain a new phase: “martensite”. Martensite is very hard and has a lot of pressure. Martensite is essentially ferrite with too much carbon inside. so we call it the new martensite phase.
Twisted crystals are very hard but brittle. Swords, knives and other tools are made from martensitic steel for high strength.
But if you look at a phase diagram, you won’t see martensite. Martensite is not a thermodynamically stable phase, but you can still get it in the final product. The graph that tells you about austenite is called a time-temperature transformation graph, or TTT curve. We will discuss TTT charts later.
So we learned that quenching can make the grains smaller and create a more stressed phase. These two effects make the steel stronger but less ductile.
In some cases, quenching can make the material more ductile and flexible. In the case of most steels, you will see that the quenching rate results in metastable martensite and not ferrite, which is thermodynamically favorable.
However, in other materials (or certain particular steels), quenching makes it possible to avoid the strengthening or weakening phase.
When it comes to superalloys, nickel-based superalloys have a very good phase called gamma’ (pronounced “gamma-primeˮ). This is the thermodynamically stable phase, but there are other “bad” phases called TCP. Technically, these make the superalloy stronger, but they also make it more brittle/less ductile.
Typically, materials scientists refine strength (the force a material can withstand) and ductility (the amount of deformation a material can withstand). TCP is slightly stronger but more fragile, so in 99% of applications, γ’ is better than TCP.
When you make an alloy at high temperatures, if you cool it slowly, TCPs can form. They are generally stable at temperatures above γ’. Unfortunately, once the superalloy is cold enough, where γ’ is more stable than TCP, diffusion will be quite slow and TCP will last indefinitely.
However, if the superalloy is quenched, this temperature range is ignored, in which TCP is stable and only γ’ is obtained.
Tempering is also used for thermal tempering of glass. Yes, the term is strange because we usually use the word “temper” to refer to the weakening of a metal after tempering, but thermal tempering is a method of making glass stronger.
2. What is the history of tempering?
Quenching existed long before scientists knew how it worked. Don’t feel bad if you don’t know how to quench your thirst either! Old blacksmiths and blacksmiths learned techniques through trial and error and held their skills closely. Some legends claim that great blacksmiths would dip their swords into the bodies of slaves (actually this might work, or at least blood might sometimes be better than water, see section on means of extinction below) .
Tempering a sword creates enormous pressure on the sword. Often, imperfect swords break. Different quenching methods or media can be used to reduce the risk of steel fracture, but until recently this level of metallurgy was shrouded in mysticism.
Perhaps the first written mention of extinction is in Homer’s The Odyssey. Blacksmiths place an ax or ax head in cold water to quench it – as this is what gives iron its strength. Quenching has been an important aspect of swordsmanship for centuries, but Japanese swordsmiths have perhaps the most sophisticated quenching techniques.
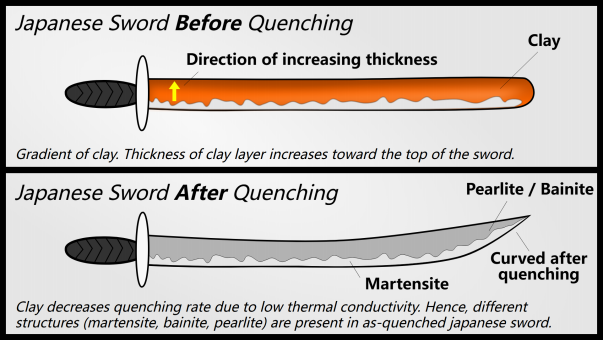
Japanese swords are made by gradual hardening. They coated the blade with clay so that the back of the sword hardened more slowly, giving it a stronger ferrite. The edge of the sword is made of pure and hard martensite, while the core of the sword is stronger and more malleable.
3. What is a quenching medium?
As you will learn in the TTT curve section below, different quench rates will produce different results. Different metals can survive different quenching without cracking.
In some steels you may want a different ratio of martensite to ferrite. Of course, non-steel metals behave very differently from steel and require their own quenching methods. Most of the time, quenching simply depends on how quickly the material is cooled.
Often the only way to control the rate of cooling is to use a quenching agent (i.e., the elements contained in the barrel). Theoretically you can change the temperature of the quenching medium, but you already have hot metal, so a few degrees change in water temperature doesn’t matter. On the other hand, differences in specific heat or boiling point can cause large differences in cooling rates.

The most common quenching fluids are water, brine (brine), oil, liquid nitrogen and air. Each of these media has different advantages and disadvantages.
01water
Water is one of the most common quenching fluids because it is readily available and allows for rapid quenching. Water is nonflammable and has a high specific heat and heat of vaporization. So when the water boils, it quickly cools the material. However, the bubbles produced by boiling reduce thermal conductivity, ultimately resulting in slower quenching. (This layer of insulation caused by the bubbles is called the Leidenfrost effect).
02 salt water
Brine is just water with salt added. It has many of the same benefits as water, but salt increases the boiling point of water, which reduces boiling bubbles and speeds up quenching. A disadvantage of brine quenching is that the salt can sometimes corrode or otherwise react with certain alloys.
The Legend About Quenching Swords in Blood: Since blood is also water with dissolved electrolytes (salts), blood is similar to weak salt water from a quenching standpoint (although urine looks more like salt water). Blood also contains large amounts of organic compounds that can clot and adhere to the slide, reducing the Leidenfrost effect. It’s also possible that these carbon molecules react to form small amounts of carbides on the surface, although I doubt this will be noticeable. In short, quenching in blood may produce different/more ideal quenching than that in water.
03 oil
There are several types of oils, but they all have lower specific heats than water, resulting in slower quenching rates. I’ve seen amateur knife makers use machine oil, vegetable oil, peanut oil – even recycled frying oil from some students’ favorite fried chicken restaurants!
Oil is a good medium speed quenching agent and can help prevent cracking. A disadvantage of oil quenching is that the oil surface can catch fire. Therefore, great care must be taken when oil quenching.
04 liquid nitrogen
Liquid nitrogen actually goes out more slowly than water because nitrogen becomes a gas (lower thermal conductivity) and has a lower heat capacity and heat of vaporization. However, liquid nitrogen ultimately results in a colder final quench than other media, which is necessary in some steel alloys. For example, many stainless steels precipitate martensite at very low temperatures that water cannot reach.
05 aerial
Typically, air quenching is accomplished by blowing cold air onto the sample very quickly. Air quenching is often used in industrial settings because air is very cheap and by controlling the speed of the air in different parts of the product you can achieve different quench rates in different locations. Air quenching is generally the slowest way of quenching.
Another type of air quenching simply allows the part to cool in still air. I generally call this “air quenched” rather than air quenched, but some alloys can achieve a quenched microstructure even at very slow cooling rates – in which case this term “air quenched” is appropriate .
4. What happens when steel is quenched?
This is the phase diagram of steel. The x-axis shows the percentage of carbon and the y-axis shows the temperature.
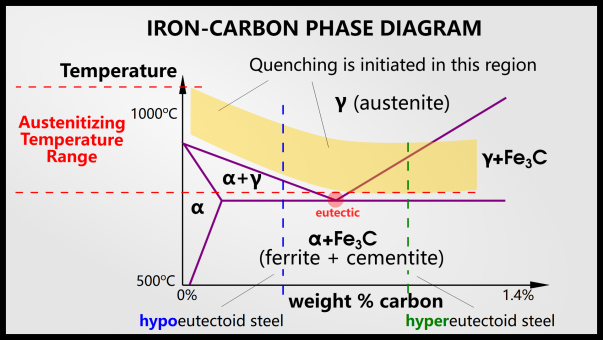
When quenching steel, we must first heat it above the austenitizing temperature. In hypoeutectoid steel (to the left of the eutectic point), the steel will have an austenitic phase. In hypereutectoid steel (to the right of the eutectic point), the steel will have two phases: austenite and cementite.
In order to obtain martensite, the steel must change phase during quenching. Below the austenitizing temperature, nothing happens during quenching. Austenite is the face-centered cubic (FCC) form of iron, usually denoted by γ, and can dissolve more carbon atoms than the face-centered cubic (BCC) form of ferrite (denoted α). Austenite can dissolve 2% of carbon, while ferrite can dissolve 0.025% of carbon. This is due to the size and number of interstitial sites in the FCC and BCC structures.
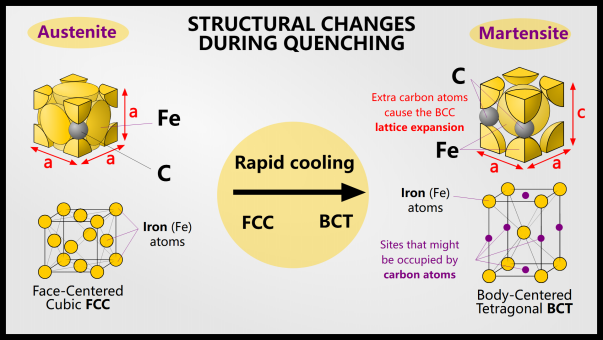
When we quench the steel, it cools quickly and hopefully transforms from BCC austenite to FCC ferrite. However, the cooling rate is too fast for the carbon atoms to move away, so they are essentially trapped in the FCC stage.
FCC iron containing carbon is no longer called ferrite: it is now martensite!
Martensite is a supersaturated solution of carbon that twists the body-centered cubic lattice into a body-centered tetragonal lattice. Depending on the steel composition and quenching rate, the final product may also contain retained ferrite or austenite.
5. What are the characteristics of the martensitic transformation?
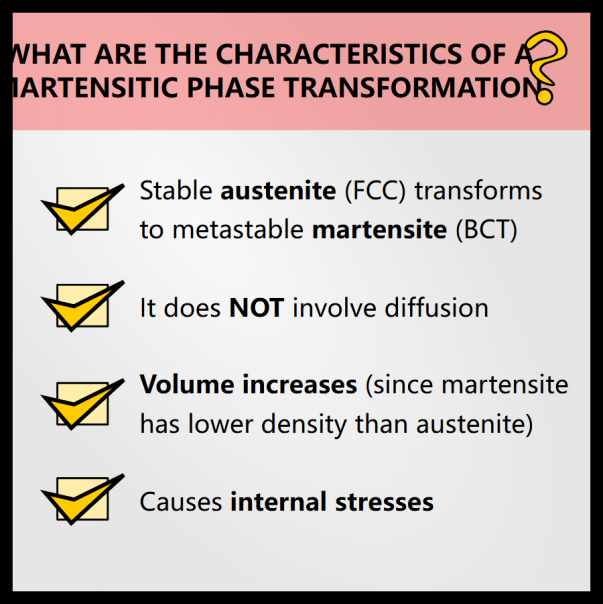
These types of phase changes do not involve diffusion. All the atoms are moving in the same direction at the same time. Martensite often forms structures that resemble plates or needles. These are called slats.
When martensite forms, it creates internal stresses in the steel. These constraints inhibit other phase transformations. Remember that a phase diagram actually has 3 axes: composition, temperature and pressure. In most materials science phase diagrams, we only represent composition and temperature, because we assume that the pressure is simply atmospheric pressure, but that internal stresses act in the same way as external pressures.
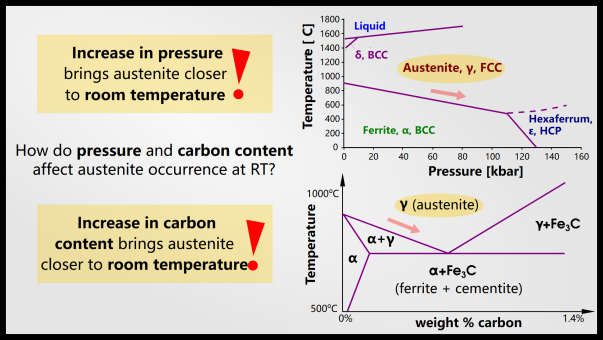
The newly formed maretnist exerts compressive stress on the remaining austenite. This is what causes “retained austenite”. There is almost always some retained austenite, so 100% steel will never become pure martensite.
Austenite is denser than martensite so the volume increases after quenching (this is why Japanese swords are curved: there is more martensite on the edge, so one part of the blade expands more than the others , which gives a curved appearance).
Large steel parts can even crack during quenching due to internal stresses caused by the volume expansion of martensite. This phenomenon is a particularly serious problem if the carbon content is greater than 0.5% by weight.
If the steel is quenched too slowly to obtain martensite, you will obtain bainite or pearlite. Bainite occurs when carbon atoms are able to partially diffuse out of the crystal lattice. Once complete diffusion is achieved, perlite appears.
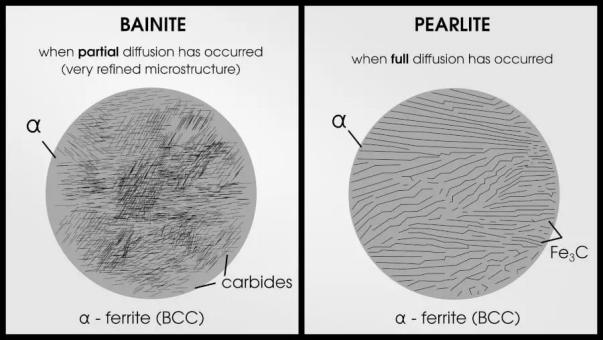
6. What are the main characteristics of martensite?
Martensite is the end product of conventional steel quenching. It is a supersaturated solid solution of carbon in a body-centered tetragonal (BCT) crystal structure. A BCT is essentially just a BCC but elongated in one direction. Martensite is a BCT because the carbon atoms are in the interstitial sites, but because the carbon atoms are larger than regular BCC interstitial sites, the lattice must be twisted.
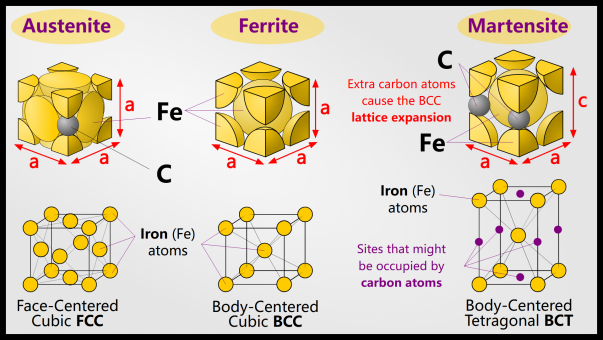
Martensite is a metastable phase, meaning it cannot be predicted thermodynamically and does not appear on any phase diagrams. Another name for metastable phases is nonequilibrium structures. Given enough time, martensite will eventually break down, but this takes thousands of years at room temperature.
However, if the martensite is heated, the iron and carbon atoms will gain enough energy to increase the diffusion rate, thereby causing the atoms to reorganize into the more stable ferrite phase.
Martensite is very hard and brittle. In fact, quenched martensite is too brittle to be used in most engineering applications. Typically, steel is tempered after quenching. Quenching is a process in which steel is reheated (below the austenitizing temperature) and cooled slowly to reduce some internal stresses.
7. What is retained austenite?
Retained austenite is actually a stable (sometimes metastable) state at room temperature, however, it does not appear on a phase diagram because conventional phase diagrams assume standard atmospheric pressure.
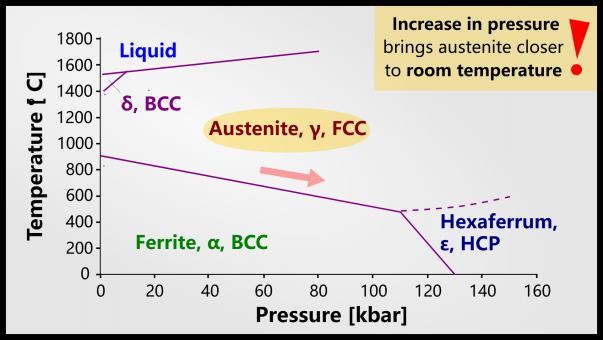
Austenite becomes more likely as pressure increases due to internal stresses caused by volume expansion of martensite. You can see the phase diagram above, for pure iron, which shows that increasing pressure prefers austenite. With the addition of carbon, austenite is also stable at lower temperatures. If the austenite remains stable until room temperature (depending on the composition of the steel), it will be an equilibrium phase. However, even though retained austenite is stable at high pressures and hundreds of degrees Celsius, in many cases the low rate of diffusion at lower temperatures will allow metastable retained austenite to persist indefinitely.
Generally, retained austenite is an undesirable microstructural feature because it is much softer than martensite. As the carbon content increases, austenite retention becomes increasingly likely.
A simple way to test for the presence of retained austenite/martensite is to use X-ray diffraction (XRD). Since martensite is BCT and austenite is FCC, their different lattice parameters are easily displayed on XRD. The integration of the curve can even provide quantitative values for the fractions of martensite and austenite retained.
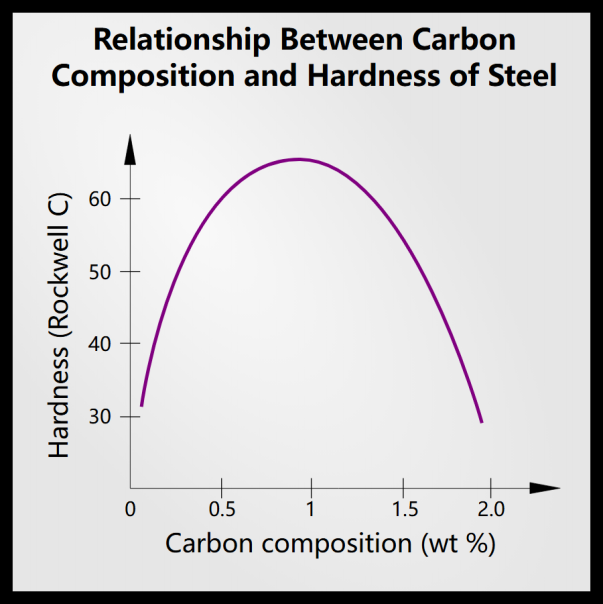
8. How does carbon content affect the hardness of quenched steel?
Generally speaking, more carbon makes steel harder and more brittle. However, more carbon also results in more retained austenite. Additionally, changes in carbon percentage alter the shape of the martensite laths and increase microcracks.
Beyond a certain point, adding carbon weakens the steel, because in unalloyed carbon steel the maximum hardness quenched in salt water is about 1% carbon.
9. What are the stages of quenching?
For reference, here is the relevant part of the iron-carbon phase diagram.
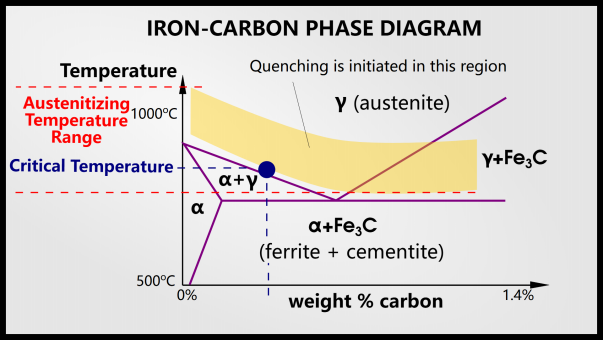
First, the alloy is heated to 30-50°C above the critical temperature. This area is shown in the image above. We don’t want to stay at this temperature for too long as it could cause the grains to grow.
If you are working with an alloy sensitive to oxidation, you may want to heat the alloy under vacuum. Some furnaces can heat under vacuum, but a simpler (small scale) method is to encapsulate the alloy in a quartz tube that has been evacuated or filled with an inert gas such as argon.

The alloy must be cooled quickly. The main way to control the cooling rate is to use different quenching media. Brine is generally the quickest practical way of quenching. Liquid nitrogen is a relatively slow quenching medium due to its low thermal conductivity and low specific heat.
If the alloy cools too quickly, it may crack. If it cools too slowly, you may not get many metastable phases. The best way to determine the optimal quench rate for a material is to use a Time-Temperature-Transition (TTT) chart.
10.What is the TTT curve?
A time-temperature transformation (TTT) plot is a diagram that shows which phases (including metastable phases) will be present at a specific cooling rate.
Temperature is displayed on the y-axis and time is displayed on the x-axis. Different stages occur, usually with a feature called a “nose.”
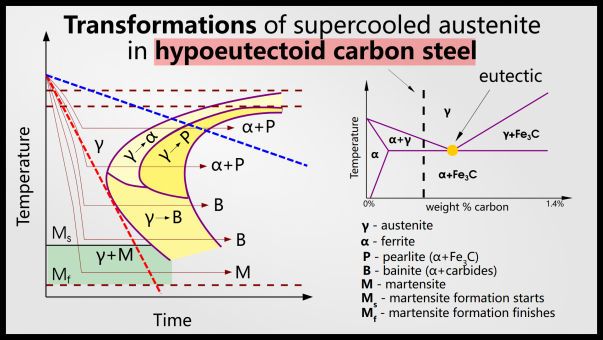
The lower horizontal line represents the time of martensite precipitation. If you dip fast enough to miss the nose (red line), you won’t precipitate any phases other than martensite. If you quench slower, as shown in the blue line, you get other phases like ferrite and cementite (we call the mixture of ferrite and cementite “pearlite”.
The purple line represents the minimum quench rate required to precipitate only martensite. In this case, that means quenching to 500°C in 5 seconds. Higher quenching rates have no effect on the fraction of phases present, but faster quenching can result in excessive internal stresses and cracking.
If the steel is not quenched quickly enough, this can result in the formation of bainite or pearlite (these are not necessarily bad phases: they are weaker but stronger than martensite).
11. Final Thoughts
Quenching is one of the most important tools for engineering alloys, especially steel. Quenching is accomplished by heating the metal and rapidly cooling it in a quenching medium such as water or oil.
Proper quenching allows precise control of the final microstructure and phases present in the alloy.
Daguang focuses on providing solutions such as precision CNC machining services (3-axis, 4-axis, 5-axis machining), CNC milling, 3D printing and rapid prototyping services.


















