So-called personalized medicine approaches are improving a little more every day and will beIts growth is achieved using 3D technology. Currently, one of the areas of application of these technologies is undoubtedly medical, where they are used to develop prostheses, implants and perhaps even 3D bioprinted organs. A major development in this area is also 3D printing of medicines, another major advance that brings us closer to more patient-friendly medicines and could fundamentally change medical approaches. Today we will focus on some of the key questions surrounding 3D printed pharmaceutical manufacturing: What are the current production technologies? Where are pharmaceutical products printed today? Of course, what does their launch mean for the pharmaceutical industry?
Today, millions of people use prescription medications daily to treat a variety of symptoms. Due to the mass production of capsules and pills, the dose we take is often higher than the recommended dose. for manufacturingThe team at Multiply Labs, the manufacturer of the drug filament for the 3D capsules, said that those affected by these overdoses are mainly children and women: “Currently, the drug is developed exclusively for white adult males, which means that all girls are responsible for their bodies, there are excessive demands,” Fred Paretti, CEO of the startup, told us. The statement confirms the importance of the emergence of personalized medicine and emphasizes the individuality of each patient In fact, an incorrect dosage of certain active ingredients can even lead to the failure of certain treatments.
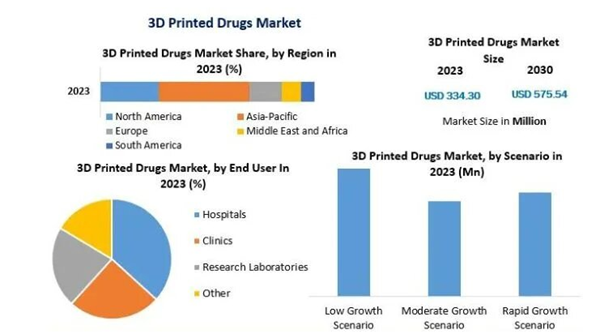
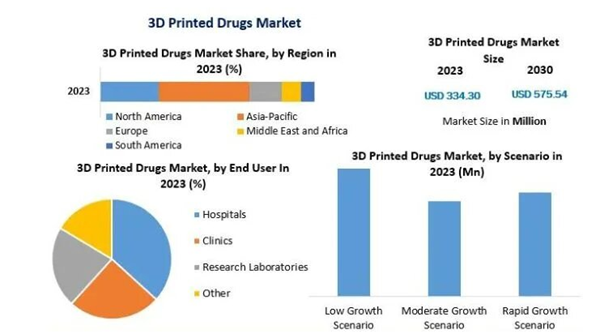
3D Printing Pharmaceutical Market Statistics 2023. The market is expected to grow at approximately 8% annually until 2030 (Image Source: Maximize Market Research).
If we look carefullyWith the development of 3D printed medicines, we see an encouraging future. According to a study by Maximize Market Research, the global 3D printed pharmaceuticals market will be worth US$ 334.3 million in 2023. However, the annual growth is expected to be 8-9% over the next few years, so the market size is expected to reach 3%. It is expected to reach $575.54 million by 2030. These estimates indicate that many changes are happening in the field, driven by the research and development of 3D technology.
The origins of 3D printed medicines
In 2015, the first 3D printed drug was launched: Spritam, a pill made using powder bed technology to treat epilepsy. It is also the first drug to receive FDA approval. This first-of-its-kind development by Aprecia Pharmaceuticals opens the door to creating personalized pills that can provide different dosages for each patient. Today, Aprecia is one of the major players in the sector; the company continues to produce the drug using its patented ZipDose® technology, which allows the drug to dissolve in seconds, a benefit for people with difficulty swallowing.

Spritam is the first 3D printed drug on the market (Photo credit: Aprecia Pharmaceuticals)
Based on research carried out by the National University of Singapore on the combination of several drugs in the same capsule, the startupMultiply Labs began producing drug filaments for 3D printing timed-release pills in 2016. Although the startup initially planned to produce personalized drugs to treat cancer, it ended up focusing on producing supplements food products printed in 3D due to the reluctance of the Medicines Agency.
Traditional pharmaceutical companies are also turning to new technologies. Darmstadt-based Merck, one of the world’s oldest pharmaceutical and chemical companies, orderedAMCM 2020, a subsidiary of EOS, uses SLS technology to produce tailor-made medicines. Merck sees huge potential in 3D printing for personalization and rapid, inexpensive production of these drugs.
Founded in 2015, Triastek has also developed these potentials as a hobby. As early as 2022, the 3D printed cardiovascular drug T20 has been approved by the FDA and has entered the clinical research and testing phase. Recently (2024), the gastric retention product T22 also received marketing authorization, becoming the first product of its kind.
SinceSince the advent of 3D printed medicines, more and more institutions and pharmaceutical companies have become interested in them and today there are a large number of startups and innovative companies using 3D printing to develop medicines. personalized. This is also due to technological progress. So let’s see how these drugs are printed!

Multiply Labs’ drug allows programmed release of substances (Photo credit: Multiply Labs)
used3D printing technology
SinceSince the release of the first 3D printed medicine in 2015, the development of its 3D manufacturing technology has continued to evolve. Taking advantage of the already known bases of 3D printing technologies, the pharmaceutical industry has been able to adapt and use them. To identify these technologies, we relied on the collaboration of FabRx, a pharmaceutical biotechnology spin-out from University College London (UCL) specializing in 3D printing of oral dosage forms via additive manufacturing, and in particular on the company scientist, Patricija Januskaite.
molten material deposit(FDM)
The FDM/FFF process is one of the most used processes in pharmaceutical 3D printing. Filaments loaded with medicinal substances can be used to make pills. A big challenge of this technology is adjusting the extrusion temperature so that the active ingredient in each pill is not affected. “The FDM process makes it possible to produce combinations of several drugs (polyps) as well as sustained or delayed release tablets,” explains Patricija.
In addition to techniques for loading filaments with drugs, it is also possible to use drug filaments that do not affect the contained drugs, e.g.An example of development from Multiply Labs, which also makes it possible to create controlled-release capsules: “We can 3D print a very thin-walled compartment capable of releasing the product in around 30 minutes, then add another capable of releasing a other medicine. in 2 hours, all in a single capsule. We can add up to four or five separate compartments in the same capsule,” added Fred.
Complex formulations can now be developed to enable rapid dissolution of pharmaceutical materials. SO,The Deglumed 2023 project uses extrusion methods to develop drugs for dysphagia patients. The filaments used dissolve quickly in the mouth and the tablets are easy to swallow.
Direct powder extrusion
This technique is similar to the development of the firstThe technology used to 3D print the ZipDose® medication. This is mainly used to make drugs with high drug loading and high disintegration due to the porosity of the material. Direct powder extrusion itself is patented by FabRx. This involves using a single screw extruder to extrude powdered material (a mixture of active ingredients and excipients) through a nozzle. The drug is available in sustained or delayed release doses, according to the British Pharmaceutical Industry.
stereolithography(YEARS)
stereolithography orSLA uses a heat source to cure a liquid photopolymer or resin. Using this technology, drugs can be integrated into polymer networks to produce pills containing active ingredients or to develop sustained-release medical devices. This technology is best for combining different drugs in the same 3D capsule.
Selective laser sintering (SLS technology)
to useSLS technology to create 3D pills involves mixing active ingredients with certain copolymers and then using lasers to fuse them together. Using this technology, drugs can be created with various properties: they can range from controlled release dosage forms to orodispersible dosage forms. Professor Simon Gaisford, Head of the Department of Pharmacy at University College London and co-founder of FabRx, explains: “Selective laser sintering creates huge potential in the pharmaceutical industry. It makes it possible to manufacture tablets without binder (like the ‘. binder jetting process tablets used in).

to useSintratec’s SLS process for 3D printing of pharmaceutical products (Photo credit: Sintratec)
Material jet printing
Although this technique is reminiscent2D printing method, but it is also similar to powder bonding technology. In pharmaceutical manufacturing, a combination of active ingredient and excipient or ink is sprayed onto a printing plate via a nozzle. They are then solidified using a powder matrix, which is then crushed. In 2019, researchers at the University of East Anglia developed a material jet 3D printing process for hot-melt droplets, in which the droplets are deposited by an extruder equipped with a pulsing nozzle and controlled by a piezoelectric system . This year (2024), another technology based on material jetting has attracted attention, namely multi-material inkjet 3D printing (MM-IJ3DP) developed by the Additive Manufacturing Center of the University of Nottingham. The soluble polymer ink (PolyACMO) solidifies when exposed to UV light and forms a matrix of water-soluble active substance within the tablet.
In addition to the technologies mentioned above, some of them vary, and many pharmaceutical companies are developing their own technologies or improving existing technologies. More and more young companies are embarking on this adventure, like the French startupMB Therapeutics.

Many companies, e.g.Craft Health is not only developing its own drug printing technology, but also its own 3D printer (Photo credit: Craft Health)
Benefits and Challenges
The benefits of 3D printing medicines are numerous, with the main goal being personalized medical care. On the one hand, the color, taste and texture of 3D printed pills can be adjusted so that taking them is no longer associated with negative experiences. This particularly concerns children and people with difficulty swallowing. Medication intake can be actively improved by using 3D printing to design pills and using easy-to-swallow materials. On the other hand, its advantages also lie in the flexibility of the mode of action and the release of the active ingredients. Typically, the goal of 3D printed tablets is to release the active ingredient at a specific time, or even multiple times, in order to maintain a constant level of active ingredient. Affected people then receive a continuous supply, even if they only have to swallow one tablet per day. Medicines can also combine different active ingredients. This helps combat polypharmacy, a problem linked to the need to take one tablet for each active substance.
However, many challenges remain and projects fail as a result. Sometimes these are technical in nature, such as certain active substances that cannot be released during the printing process. Regulation is a bigger barrier. Generally speaking, medicines are subject to the Medicines Act and its strict rules. Each individually printed pill poses a problem for the Medicines Agency because it is a separate medicine that must also be tested. This leads to endless trials and slows down research.

3D printed medications can combat polypharmacy by combining multiple active substances in a single tablet without being too bulky. (Image source: Merck)
What is the future of 3D printed medicines?
3D printed medicines are no longer a new thing on the market and the last decade has laid an important foundation for future innovation in this field. The mentioned data on the 3D printed pharmaceutical market illustrates this trend. In the coming years, more and more 3D printed drugs will conquer the pharmaceutical market and be authorized for clinical trials. As research strives to develop increasingly personalized drug production methods and 3D technology evolves in this direction, the challenge now is to accelerate the introduction of 3D printed drugs. This could make the dream of personalized medicine a reality. “In ten years, no patient will want to receive the same thing as another million people. No doctor will prescribe the same drug to two patients,” concluded Fred Paretti of Multiply Labs.
source:3dnatives
Daguang focuses on providing solutions such as precision CNC machining services (3-axis, 4-axis, 5-axis machining), CNC milling, 3D printing and rapid prototyping services.