for the human body3D printed products must be made from biocompatible materials that do not cause allergic or toxic reactions. But what does biocompatibility mean when it comes to 3D printing materials? What materials are biocompatible?
Today it is available forThere are more biocompatible material options than ever for 3D printing, allowing dental and medical professionals and a variety of manufacturers to 3D print products for short or long term skin contact (wearables, swabs COVID testing equipment, orthotics, earplugs, personal protective equipment). or products intended for use inside the human body (dental prostheses, arthroplasties, bone implants, vascular stents). Even medical device development companies use biocompatible materials to 3D print their product prototypes.
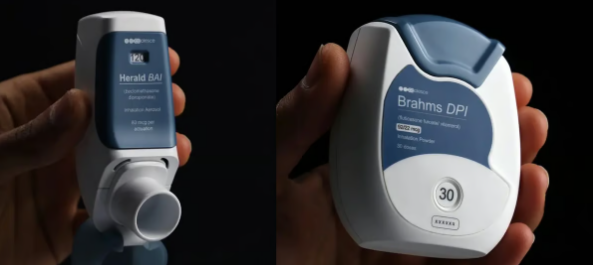
TheseThe 3D printed inhaler prototype was produced by Coalesce Product Development, the UK division of Novartis, using Formlabs 3D printers and biocompatible resins, reducing the delivery time of its inhalers by more than 80%. (Source: Formlabs).
Let’s explore biocompatibility by looking at two key application areasWith the many types and uses of 3D printing materials, these two fields can serve as models for countless other applications: dentistry and prosthetics. Later, we will discuss the materials from which permanent body implants, such as knee replacements and cranial implants, are 3D printed.
Of course, this overview of biocompatible materials is not intended to provide any form of medical advice, and please keep in mind that biocompatibility regulations vary in the United States, European Union, Asia and in India.
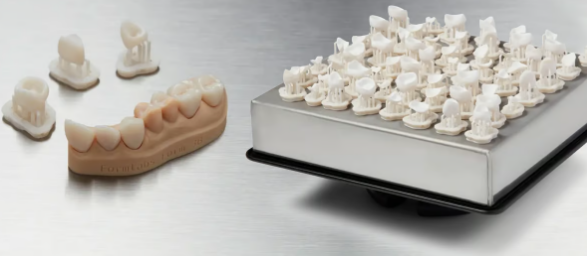
Biocompatible dental materials
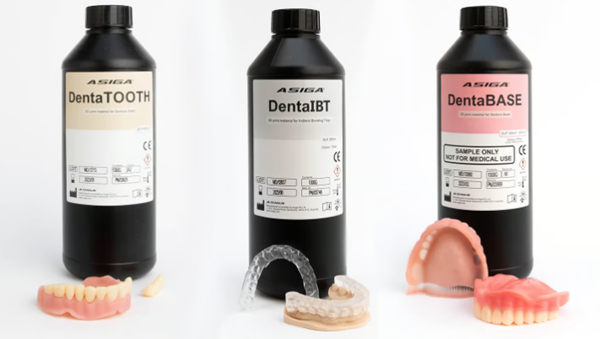
Asiga dental resin (Source: Asiga)
The entire dental industry has quickly3D printing is used in a range of products. From models to temporary dentures to permanent implants, 3D printing uses plastics and metals to provide patients with the specific products dentists need, at the speed and price consumers expect. In fact, according to Zion Market Research, the global dental 3D printing market will be worth approximately US$3.25 billion in 2021.
Dentists often use resin3D printing (also known as stereolithography) is used to produce dentures, mouthguards, surgical tools, and many other products that are placed temporarily or permanently in the mouth. There are a wide variety of resins on the market, and manufacturers have specific classifications, ratings, and industry standards that are like secret codes when it comes to biocompatibility. Let’s see what these labels and numbers actually mean.
biocompatible3D printing material categories
according toAccording to FDA regulations, the United States classifies biocompatibility as Class I, Class II or Class III (according to MDCG regulations, the European Union classifies biocompatibility as Class I, Class II or Class III). There are similar systems in other parts of the world, but with different classifications. For example, since there are no international standards, Class II products in the United States may fall into Class III in China. In this article we will focus on American and European standards.
Even if you will seeResin sold as Class I, but the material itself is not classified, but products made from this material must be subject to testing (if testing is required).
Class I medical devices pose low to moderate risk and require general controls. Nearly half of all medical devices are considered Class I and 95% are not subject to any regulatory oversight. General examples of Class I medical devices include elastic bandages and hand-held surgical instruments that only come into contact with intact skin. In terms of dental 3D printing, Class I materials are often used to make dental impression testing equipment.
Class II medical devices carry moderate to high risks and require special controls. These materials can be sold in the United States once the manufacturer has registered and listed them with the FDA and meets applicable requirements. Class II device types include removable skin staples and permanent restorations in dentistry, inlays, onlays, and dentures. These products are intended for use in contact with blood, body fluids, organs, tissues and cells.
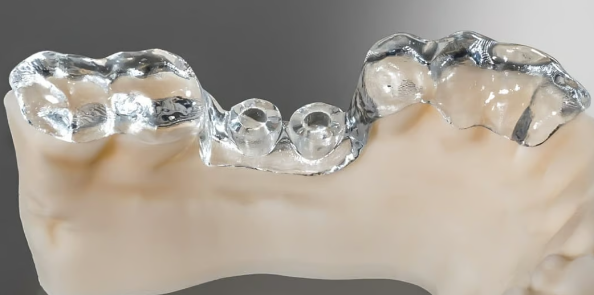
to useDental surgery guide made from Detax Freeprint Ortho resin (Source: Detax)
These two categories are then subdivided into subcategories based on exposure time (a, b and c), from finite time to long time to permanent time. For example, Class IIa means that Class II equipment has a limited useful life, specifically less than 24 hours of use. Subcategory “b” indicates prolonged use (24 hours to 30 days) and subcategory “c” indicates permanent use (more than 30 days).
Biocompatibility isn’t just a measurement, it’s the International Organization for Standardization(ISO) stipulated various measurement standards. Specifically, Standard 10993 and its subcategories represent biocompatibility types. For example, ISO 10993-5 assesses the risk that a material will be toxic (or cytotoxic) to living cells, while standard 10993-10 addresses the risk that a material will act as an irritant or sensitizer. It is also being evaluated whether the material can induce genetic mutations, cause skin rashes or cause widespread toxicity. Your 3D printing equipment may meet some or all of the standards.
The classification is based on the risk of the device, whileISO 10993 is based on the duration and type of contact with body tissues and fluids, which are the most important factors when selecting equipment materials.
Manufacturers reveal whether materials meet international biocompatibility standards after evaluation and testing. Manufacturers often even disclose the laboratory that performed the tests.
ISO has other standards worth looking into. ISO 22112 addresses the material properties of polymer or ceramic dentures, including those 3D printed. For materials for crowns and veneers based on polymers, there is the ISO 10477 standard; for polymer-based denture bases there is ISO 20795. Please note that these standards are not specific to 3D printing and also apply to materials used to mold and process dental products.
Let’s take biocompatibilityTake 3D printing resin as an example. Denture resin from 3D printer and materials maker Formlabs comes with a data sheet indicating that the material has been tested for biological evaluation of medical devices at WuXi AppTec Laboratories in St. Paul, Minnesota, and is certified biocompatible according to ISO 10993, non-mutagenic, non-cytotoxic, will not cause erythema or edema reactions, is not a sensitizer and will not cause systemic toxicity. The post-cure properties of parts made from this material comply with ISO 22112 and ISO 10477 standards. Currently, 16 of Formlabs’ materials have been publicly tested against various ISO 10993, ISO 18562 and USP Class VI parameters, with the results published on the company’s website.
you use3D printed materials must be able to deliver the specific levels provided by Formlabs in the datasheet or by contacting the manufacturer.
Another logo you can see on the materials isUSP Class VI, which refers to a set of biocompatibility testing requirements from the United States Pharmacopeia (USP), a nonprofit organization whose standards serve as a benchmark for Food and Drug decision-making Administration (FDA) of the United States. This test is one of the most common methods for determining the biocompatibility of materials. There are six levels, with Level VI being the most stringent and designed to prove that chemicals leaching from plastics will not cause harmful reactions or long-term bodily effects.
It is not uncommon to see certain terms on materials that mean nothing. For example,“FDA 510(k) Approval” or “FDA 510(k) Approval” is not actually a certification. When the FDA approves a device through a 510(k), it does not review whether the product is safe or effective for use by patients. This simply agrees with the manufacturer’s claim that the device (or materials) are similar to others already on the market. The same goes for “CE certification”. The letters CE on an EU product mean that the manufacturer certifies that the product complies with European standards for health, safety and environmental protection. It is not a quality indicator or certification mark.

Daguang focuses on providing solutions such as precision CNC machining services (3-axis, 4-axis, 5-axis machining), CNC milling, 3D printing and rapid prototyping services.